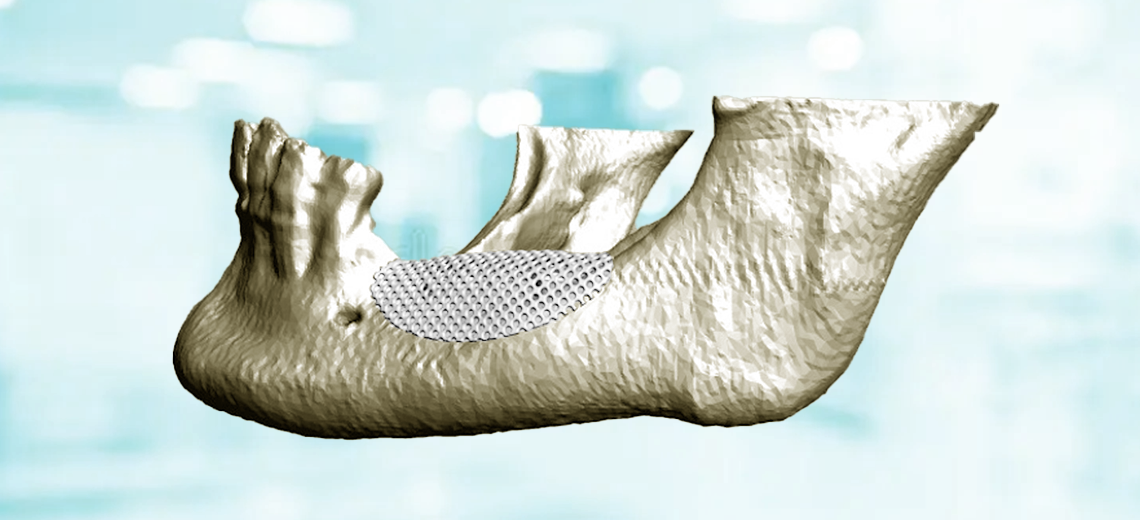
Tooth loss causes an anatomical change in the alveolar bone that requires oral rehabilitation practices using endosseous implants. However, unfavorable conditions can lead to insufficient vertical/horizontal bone size to the point of compromising the insertion of endosseous implants and the aesthetic result.
Guided bone regeneration (GBR) is a surgical technique that uses membranes and filling materials to recreate adequate regeneration of missing bone tissue, in order to insert endosseous implants in an adequate bone volume that completely surrounds the entire implant surface, and in an optimal position for subsequent prosthetic rehabilitation. Guided bone regeneration uses both absorbable and non-absorbable barrier devices (membranes), whose task is to create and maintain a protected regeneration space underneath it, within which the created graft will enjoy a local concentration of bone growth factors until completion of the regeneration.
The ideal membrane should be biocompatible, able to cover the defect while maintaining the space created, stabilize the underlying clot or graft material, allow for proper healing, and prevent the growth of connective tissue (which tends to grow faster than bone tissue) into the defect.
Researchers from the Department of Clinical and Experimental Medicine, together with colleagues from the Department of Engineering at the University of Florence and the CreaMED team, conducted a comparative study between two types of regeneration meshes, made of traditionally used titanium alloy (Ti6Al4V) and 3D printed polycaprolactone (PCL), presented at the 77th edition of the Congress of the Italian Society of Anatomy and Histology.
The aim of the study arises from clinical observation: GBRs performed with titanium mesh sooner or later tend to expose the oral mucosa which undergoes reabsorption. The mid-crestal mucosal tissue is avascular, so the first incision and subsequent application of the load can lead to tissue necrosis.
This condition occurs in 30-50% of cases even with better surgical technique. The presence of a rigid mesh material is assumed to be the cause of this condition, which is why it was decided to use PCL (bioabsorbable material, already used in orthopedics) and which was observed to have an elasticity like the oral mucosa.
The mechanical performance of these two types of meshes were tested in the study in order to avoid the failure condition.
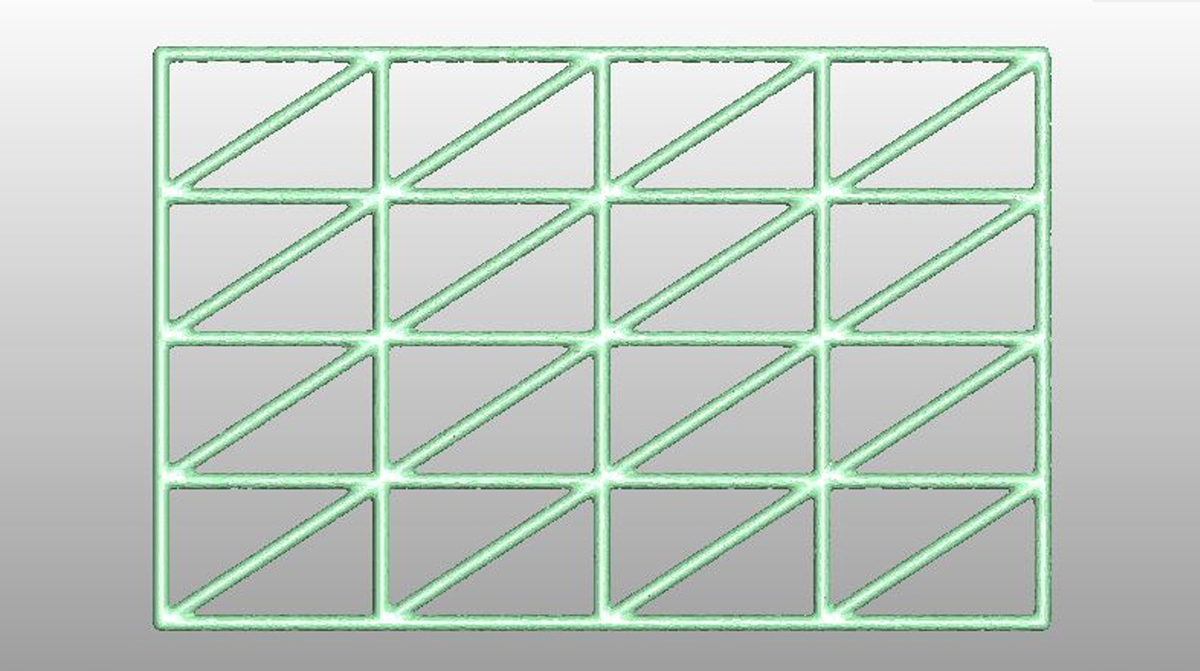
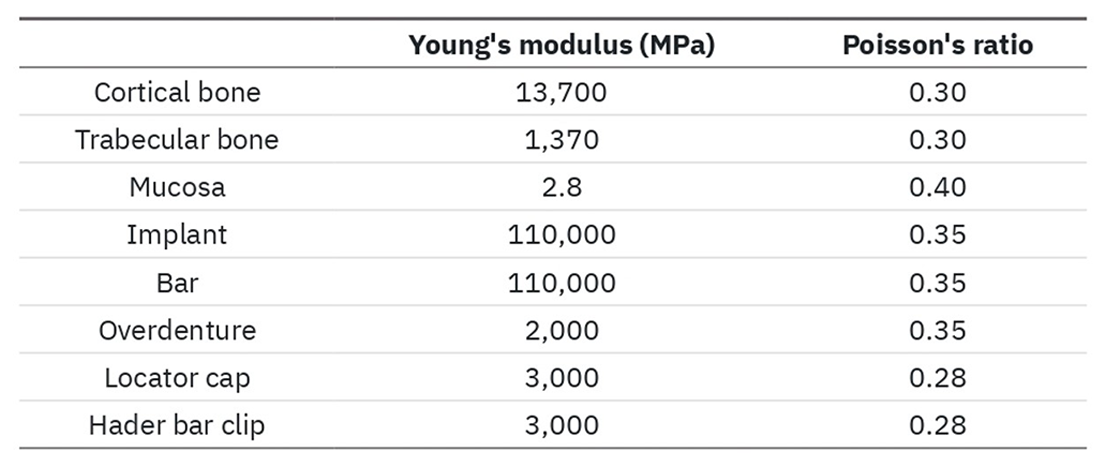
Planar mesh structures with two different thicknesses (0.8 and 0.2mm), dimensions 10x30 mm and hole width equal to 23mm2 were considered, on which the Finite Element Analysis (FEA) was appropriately set. The PCL and TI6Al4V meshes were considered linearly elastic, homogeneous and isotropic, with boundary conditions applied on the short sides and load applied axially with a spherical point of 10mm diameter from 130N at 10mm/min.
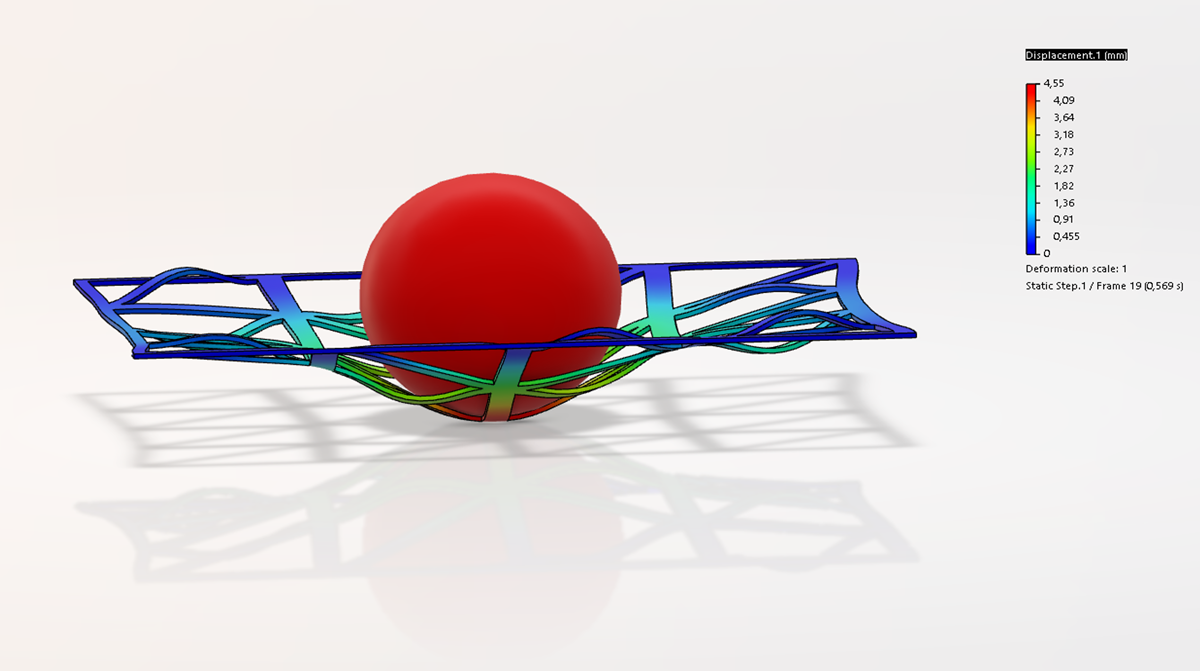
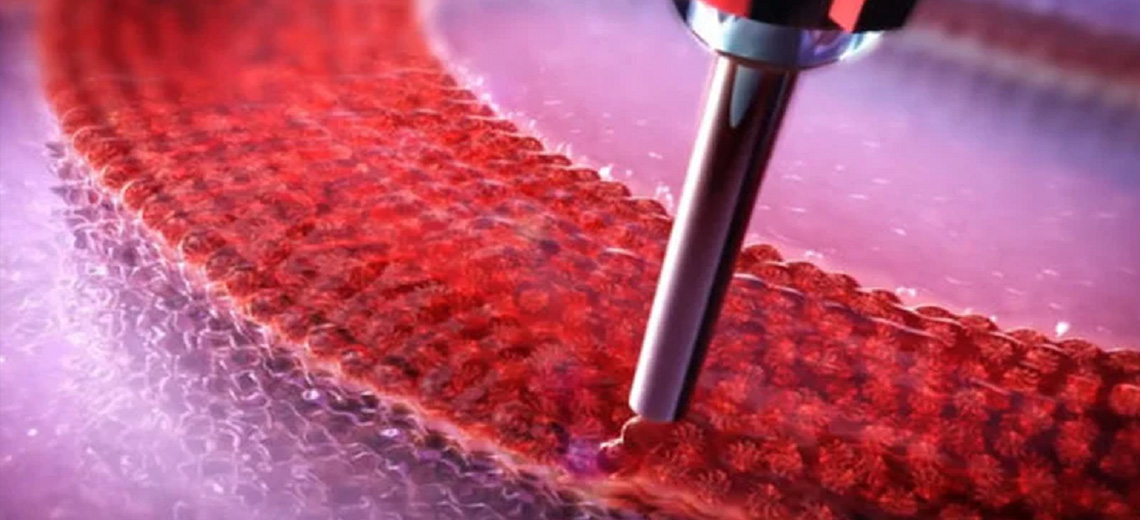
Following the FEA simulations, in vitro tests were performed on 15 meshes (10 PCL and 5 Ti6Al4V). In collaboration with CreaMED, the PCL meshes were 3D printed with FFF technology and the optimal printing parameters were defined. Subsequently, the samples were appropriately prepared and sterilized to identify any load variations.
The mechanical tests were carried out with an MTS 810 machine under the same conditions as the in-silico simulations with the application of the load until the structure fractured.


From the results obtained, it was observed that the breakage of both non-sterilized and sterilized PCL meshes occurred at very similar load values and slightly higher for the former, while the value was double in the case of Ti6Al4V meshes. It was therefore found that the stiffness values of PCL are lower than those of Ti6Al4V and comparable to those of oral mucosa reported in literature.
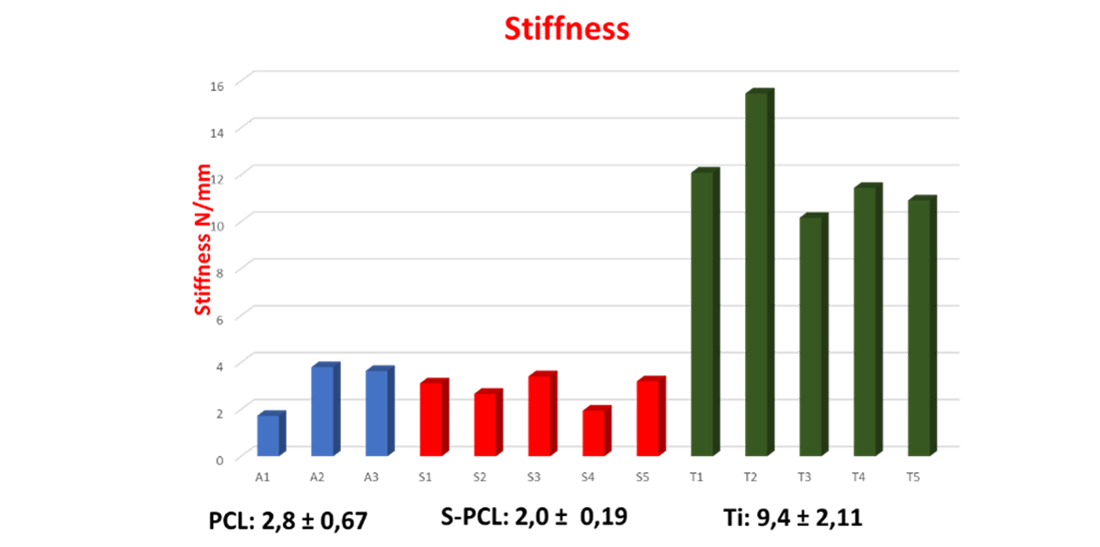
The study confirmed that the PCL mesh, compared to the traditional Ti6Al4V alloy mesh, perfectly meets the requirement for an elasticity comparable to that of mucosal tissue, such as to prevent its reabsorption and consequent failure of bone rehabilitation.
The study shows that the use of PCL mesh could avoid the occurrence of these problems and establish itself as the best candidate for GBR therapy, also thanks to the bioreabsorbability of the material and the advantage of using a production technique such as additive manufacturing (AM) which significantly reduces process times and costs, ensuring maximum flexibility in creating structures with complex geometries that are faithful to the anatomical site.
Given the positive results, in the coming months, analyses are planned on three-dimensional anatomical meshes with osteoblast cell cultures to verify any toxicity or cellular pollution from nanoplastics derived from its degradation, until continuing with in vivo studies on animals and humans.