Bacterial keratitis (BK) is an ocular infection which constitutes the majority of infectious keratitis cases all around the world, and has estimated about 2.5-799 cases per 0.1 million population per year.
If not treated properly, serious problems could arise, which could lead to severe sight-threatening complications such as ocular morbidity, corneal scarring, perforation and blindness.
Current therapy for BK involves administration of fluoroquinolone eye drops every hour for 24 hours for most patients and treatment is continued for at least 2 days depending on the severity of the patient. Current therapy based on fluoroquinolone eye drops, if administered frequently, carries a higher risk of developing multidrug resistance.
Antimicrobial peptides (AMPs) have emerged as a potential strategy to overcome the challenges of multidrug resistance. They have great advantages and are effective on a broad spectrum of bacteria. Some of them include rapid killing, anti-biofilm activity, low cytotoxicity, and minimal possibility of developing resistance in bacteria.
In this study, gelatin methacryloyl (GelMA) and chitosan methacryloyl (ChiMA) hydrogels were used for 3D printing of contact lens-like patches. They possess excellent mechanical strength and biodegradable and biocompatible properties with high oxygen permeability and porosity, which make them suitable for delivery systems for disease treatment, and they also impart mucoadhesiveness to the cornea. Moreover, chitosan, which was used in the ChiMA synthesis, is also known to exhibit antibacterial activity. All these characteristics make the above materials perfect candidates for 3D printing.
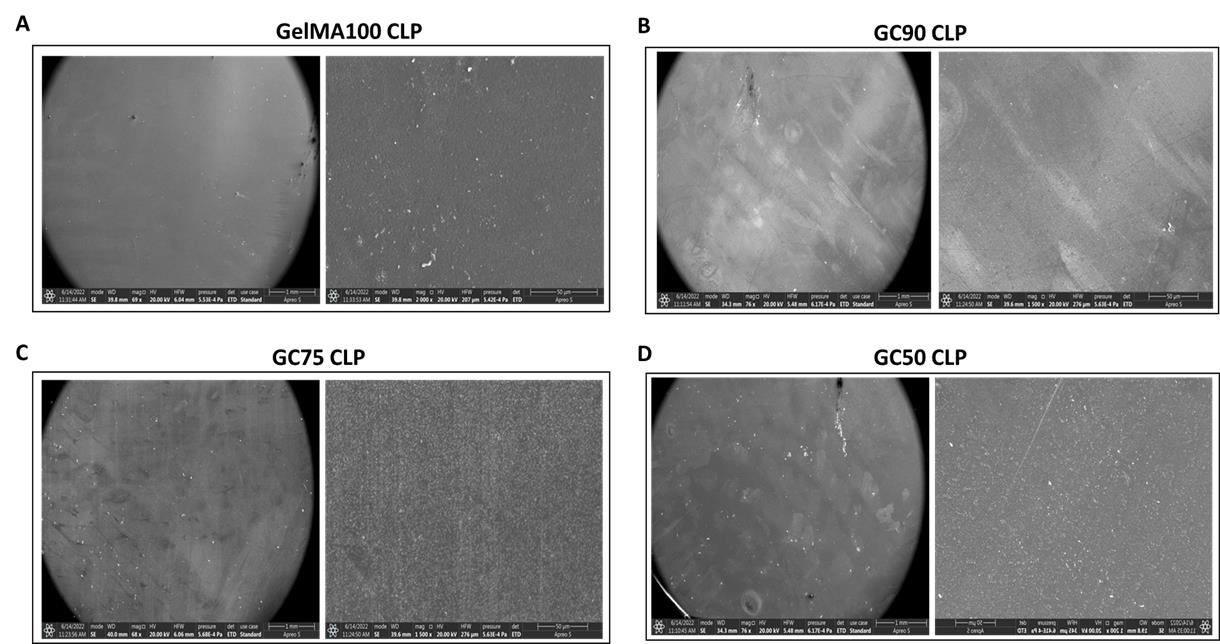
Porcine skin gelatin, chitosan, 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (Irgacure 2959), methacryloyl chloride (stabilized with MEHQ) and S100A12 peptide (AMP) were printed using the Bio X printer. Different combinations of the elements were used:
|
|
% GelMA | % ChiMA |
| G100 | 100 | 0 |
| GC90 | 90 | 10 |
| GC75 | 75 | 25 |
| GC50 | 50 | 50 |
The basic modeling values for the patches are 15 mm diameter and 0.3 mm thickness. The print head and print bed temperature were controlled at 26 and 9 °C, respectively. A 27G nozzle was used, the printing speed was set at 10 mm s−1, the pressure at 40–60 KPa, and the infill density was 80%. After printing, the CLP was exposed to UV radiation at 365 nm to photopolymerize for 2 minutes. The printed scaffolds were stored in sealed boxes at 4 °C, protected from humidity.
Hydrogels loaded with 500 μg of AMP and AMP were found to be non-toxic to HCE cells at the tested dose during the incubation time, with cell viability of 79.05 ± 0.56 (GelMA100), 80.07 ± 0.39 (GC90), 79.89 ± 0.49 (GC75), 78 ± 0.41 (GC50), and 92.51 ± 1.84% (AMP).
Cornea is particularly prone to infections because it has a moist mucosal surface and is continuously exposed to the external environment, making it vulnerable to various microbes. The hydrogels were able to destroy the monolayer of cells, and GC50 penetrated deeper into the multilayered epithelial cell layer and returned to a normal state without damaging any cells, which suggests that the prepared formulations could permeate through the cell layer and maintain cell integrity, and this is due to the presence of chitosan in the printed scaffolds. Chitosan facilitates the permeation of active ingredients by disrupting the tight intercellular junctions and increases the permeation into the cornea through intracellular absorption and intercellular pathways.
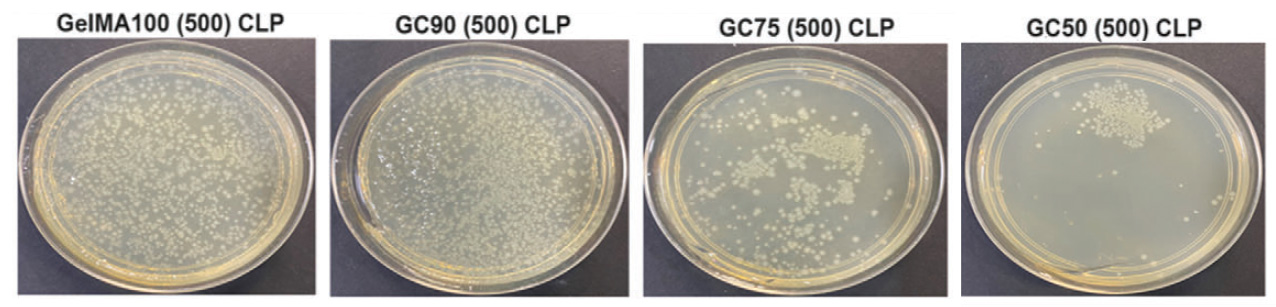
The bacterial inhibition zone of GC50 loaded with 500 μg AMP obtained by agar-plate method showed that this ink resulted in a diameter of 45 ± 0.14 mm compared to GC50 loaded with 100 μg AMP with a diameter of 38 ± 0.41 mm. GC50 CLP without AMP showed a ZOI of 18 ± 0.3 mm, indicating that chitosan present in the formulation can also inhibit bacteria.
| Formulazione | ZOI [mm] |
| GC50 CLP | 18 ± 0.3 |
| GC50 CLP (100) | 38 ± 0.4 |
| GC50 CLP (500) | 45 ± 0.1 |
Furthermore, the antibacterial efficacy of AMP (100), GelMA100 (100) and GC50 CLP (100/500 μg, AMP) was investigated in a rabbit model of bacterial keratitis.
The results indicated that GC50 (500) significantly reduced the bacterial load of the cornea. GelMA100 (100) and GC50 (100) reduced the bacterial load by two-fold compared to AMP.
The results indicated that rabbits treated with GelMA100 (100) and GC50 (100/500 μg, AMP) significantly reduced the live bacterial count compared to free peptide (used as eye drops) and the control group.
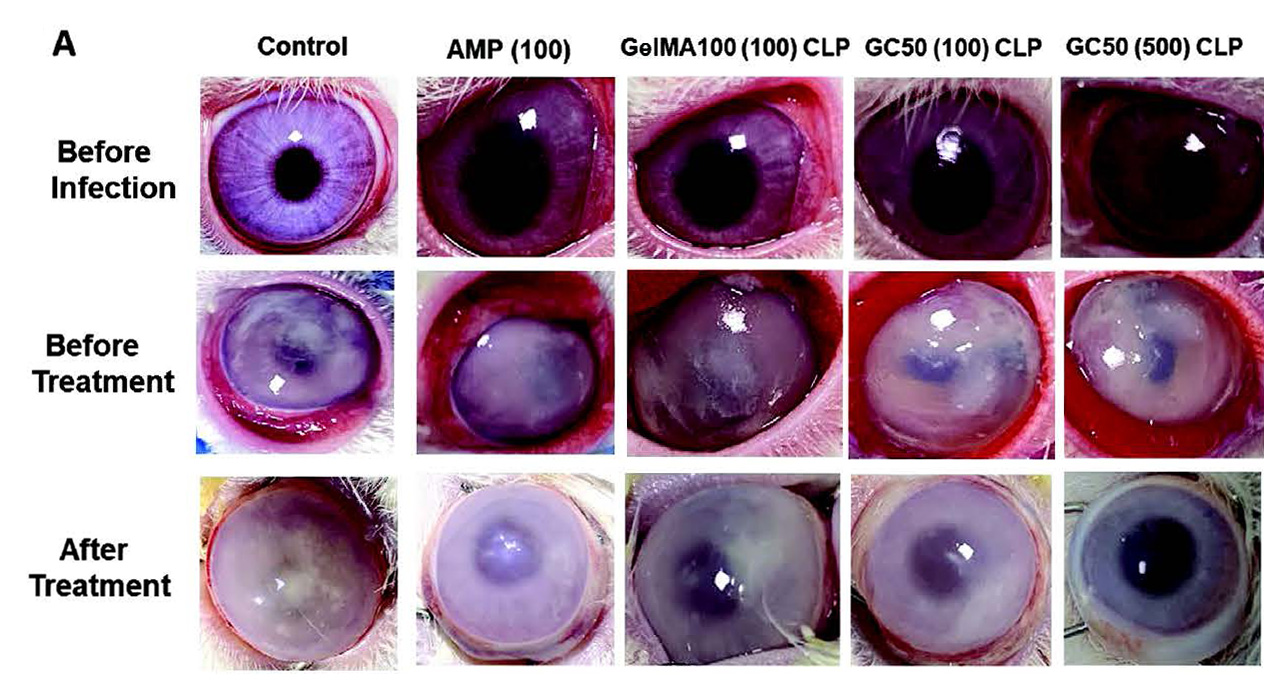
Corneal patches have adequately met the needs of the ocular environment, such as water absorption and proper transparency, in addition, they have a better retention time on the corneal surface than conventional eye drops and significantly reduce tear drainage of the therapeutic agent, thus increasing the accumulation of high concentrations of antimicrobial agents in the corneal tissue and allowing free passage of antimicrobial agents through the open pores of the lens, resulting in increased corneal penetration.
Source: Wiley online library