Skeletal muscle tissue (SMT) is the largest organ by mass in the human body and is critical for locomotion, posture, respiration, physiology, and energy homeostasis. Genetic or acquired diseases, as well as damage and aging can make this organ dysfunctional and cause significant impairment in the quality of life and human health.
Over the past three decades, several three-dimensional SMT culture systems have been developed to mimic the native muscle microenvironment and understand skeletal muscle function, plasticity, and disease.
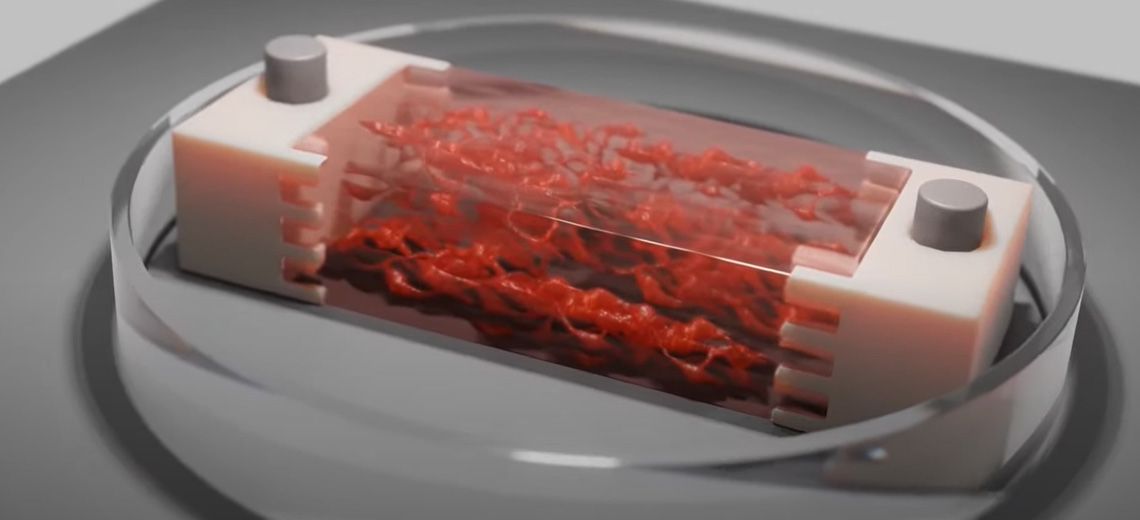
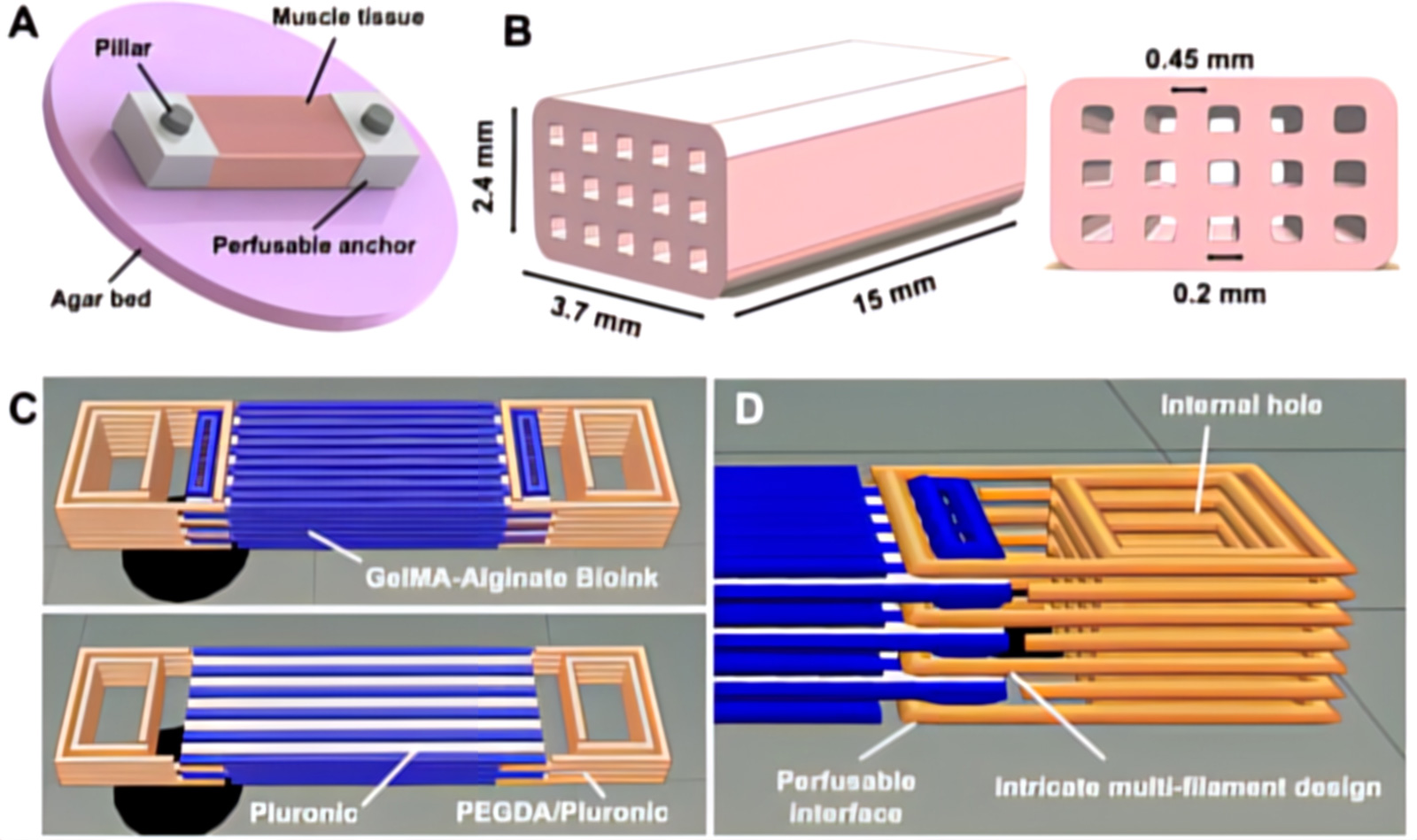
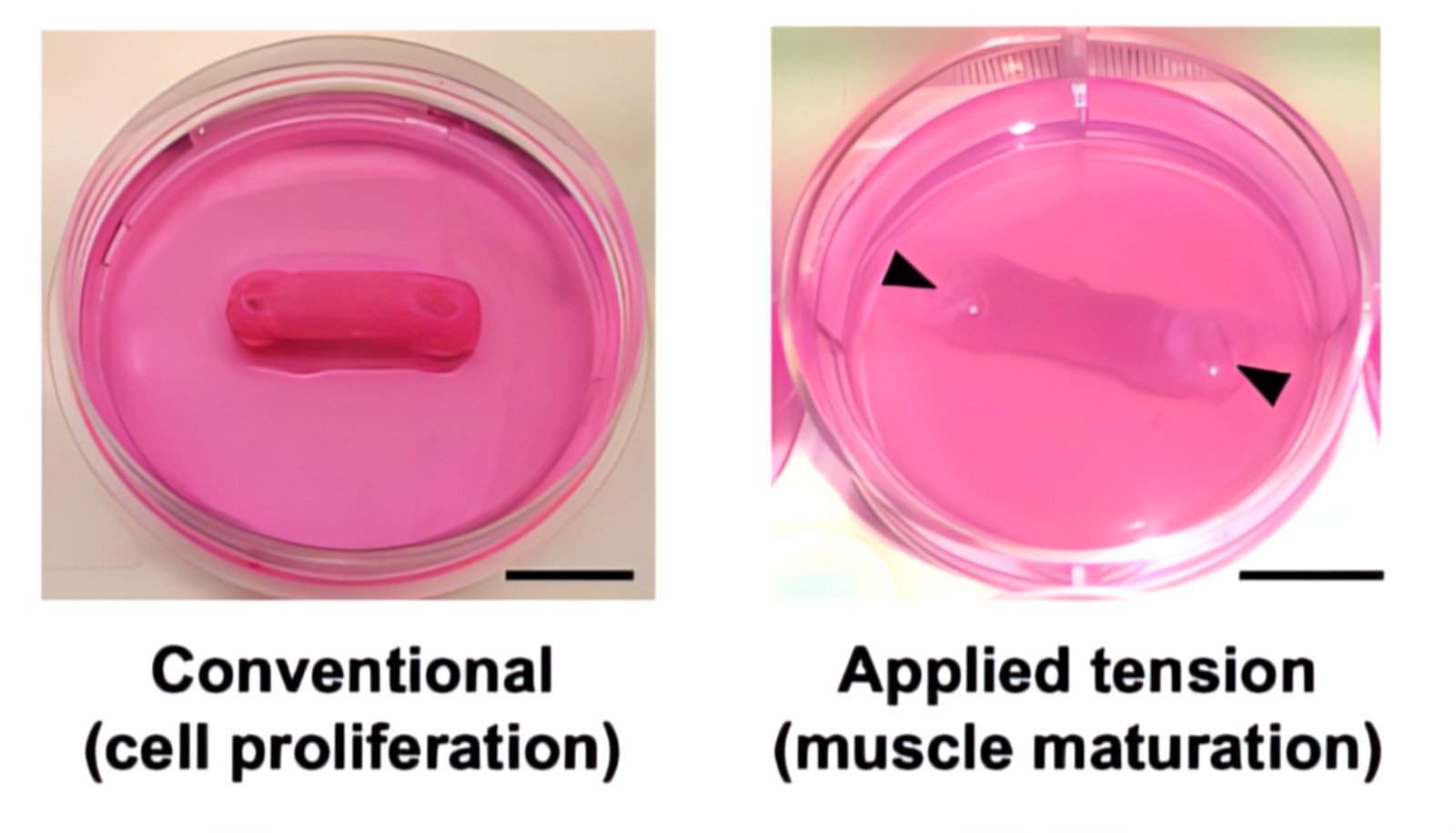
Multimaterial extrusion-based 3D bioprinting has been used to make perfusion-capable and biohybrid SMTs. To impart mechanical tension for muscle maturation, constructs were fixed during the differentiation phase to agar beds at the bottom of culture wells. The pillars were passed through the anchor holes of the SMT and drilled into the agar.
The bioprinted SMT consists of a multilayered tissue structure with a nominal size of 3.7×2.4×15 mm and was filled with internal microchannels with a diameter of 200 μm generated within the constructs by a sacrificial ink (white) composed of Pluronic F-127 printed simultaneously with the bioink based on 8% GelMA and 7% NaAlg loaded with skeletal muscle cells (25×106 cells/mL) (blue) and then removed after printing exposing the constructs at a temperature below its gelation point.
Moreover, the tissue was co-printed with integrated anchoring structures composed of a mixture of PEGDA and Pluronic F-127, which served to attach the construct to the culture templates used for tissue maturation. The anchors were designed with an internal interface capable of perfusing the core block microchannels left after removal of the sacrificial ink. To stabilize the interface, the anchor ink (orange) was shaped to intertwine with the bioink and also form a central hole that serves as an insertion point to stabilize the pillars in the maturation template.
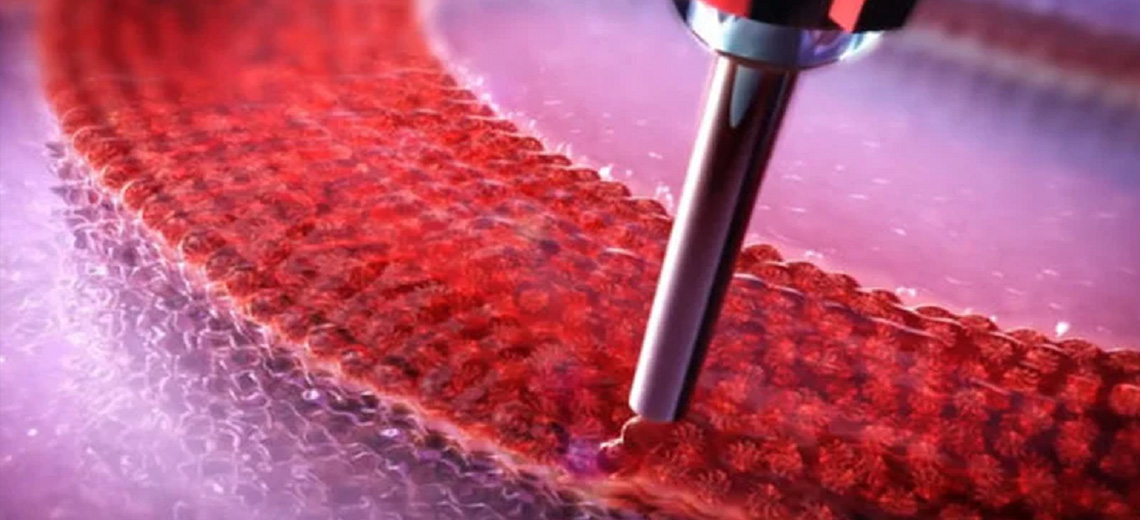
The inks were extruded through conical nozzles with an internal diameter of 410 μm (22G). To maintain both GelMA and Pluronic F-127 in their gel state, the build bed temperature was set to 19 °C. GelMA-NaAlg blends were printed with the temperature-controlled printhead and held at 21 °C for the duration of the print. The cell-loaded bioink was printed at a speed of 10 mm/s and an air pressure of 30 kPa.
The sacrificial bioink was printed at a speed of 8 mm/s at 75 kPa. To print the anchors, an ink based on PEGDA 700 and Pluronic F-127 was printed at dispensing speeds of 75 mm/min and pressure of 780 kPa. Each layer was photocrosslinked directly after printing at 365 nm for 30 seconds. After printing all seven layers, the constructs were immersed in a CaCl2 solution for 45 seconds). Because Pluronic F-127 has a low critical gelation temperature (approximately 14°C), washing the constructs with the ice-cold cross-linking agent simultaneously eliminated the sacrificial ink and cross-linked the alginate portion of the hydrogel.
The bioink was found to be soft enough to support printing and culturing of myoblasts and could be printed with 90% accuracy. The line width of the bioink and sacrificial ink are approximately 450 and 200 μm, respectively, and their coprinting allowed creating architectures close to the target design with high structural fidelity. In particular, parallel microchannels were observed running along the longitudinal axis of the constructs, open at the ends and contiguous to the external space.
Post-printing cell viability demonstrated that cells maintained high viability (>90%) in all types of bioprinted designs. This result suggests that the presence of the channel and anchor inks, as well as the related bioprinting protocols, did not substantially alter cell viability. To evaluate cell proliferation over time, entire constructs were analyzed at days 3, 6, and 15. High cell viability was found across all designs and construction conditions. However, in designs with channels (+Ch/+Anc/+Tens; +Ch/-Anc/-Tens; +Ch/+Anc/-Tens), lower amounts of dead red blood cells were detected compared to conditions without channels ( -Ch/+Anc/+Tens; -Ch/-Anc/- Tens).
Beyond 200 μm depth, constructs without channels showed dead cells distributed over a large area. In contrast, constructs with channels had fewer dying cells that were also localized within confined areas, suggesting that the presence of channels supports cell viability. Furthermore, imaging showed the effect of mechanical tension during the differentiation phase on the morphological change of cells towards the formation of myotubes. Constructs subjected to mechanical tension (+Ch/+Anc/+Tens; -Ch/+Anc/+Tens) showed strong cell alignment on days 3 and 6 and long, viable myotubes at different tissue depths (100 and 500 μm) on day 15.
In the absence of mechanical stress (+Ch/-Anc/-Tens; -Ch/-Anc/-Tens; +Ch/+Anc/-Tens), cells aligned only to a limited extent in the of early maturation (day 6) and no obvious viable myotube was visible in the later maturation phase (day 15).
At the end of the maturation process (day 15), the channeled constructs were still suitable for perfusion with faster kinetics than a solid muscle without channels. The channeled mature biohybrid constructs were completely perfused by the staining solution after only 30 minutes of staining exposure, while the constructs matured in the absence of channels were completely stained after 10 hours of staining exposure.
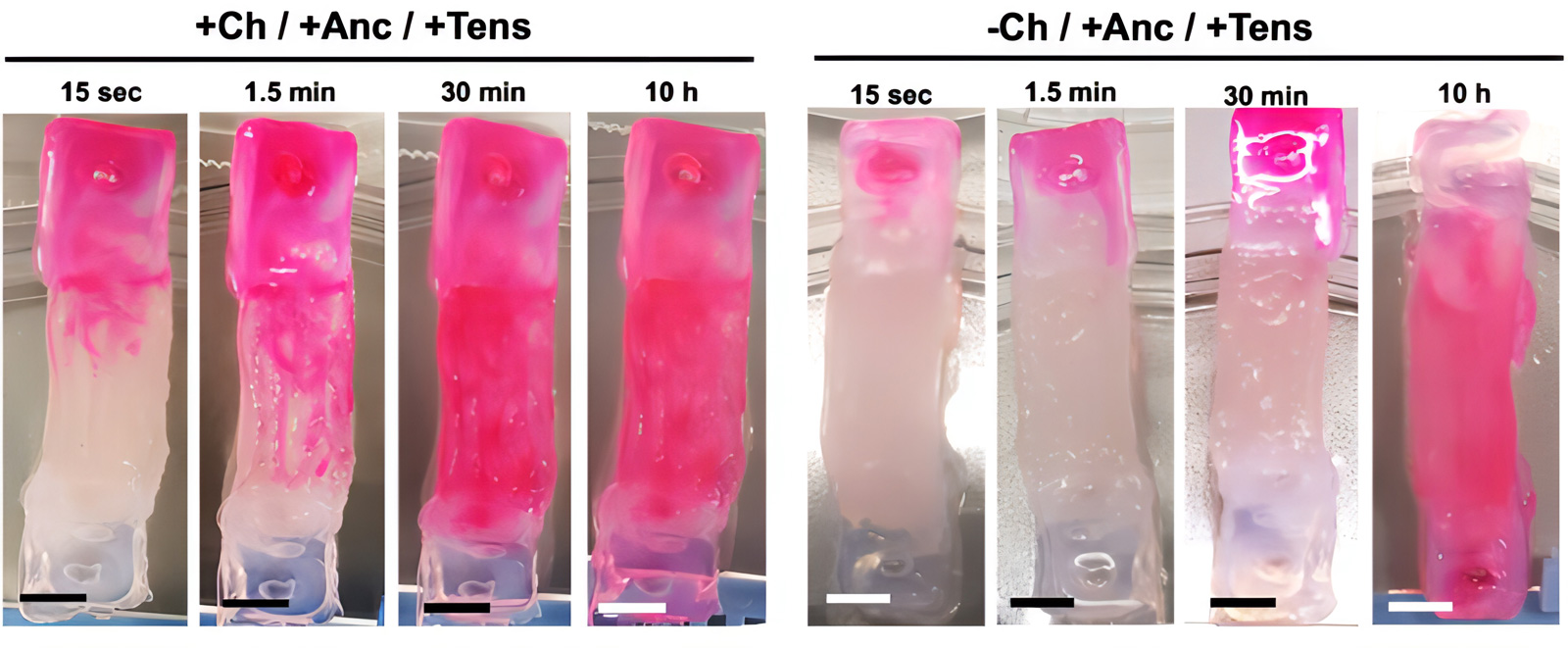
The design printed with PEGDA/Pluronic and GelMA/NaAlg was robust enough to withstand forces on the order of μN and mN, which are typical of bioactuators of similar size. Furthermore, modeling perfusion networks within the constructs not only reduced the presence of hypoxic regions without altering the structural stability of the assembly, but also provided contact guidance to growing myocytes, promoting fiber formation. muscles aligned unidirectionally.
Source: Wiley online library