The maturation of all blood cells occurs within the microenvironment of the bone marrow, a soft tissue that fills the cavities of the bones, composed of a network of extracellular matrix components and intercellular fluids generated by pressure gradients from the vascular system that facilitate the process of hematopoiesis.
Platelet disorders, which affect approximately 3 million people worldwide, include a wide range of rare diseases that can remain undiagnosed or misdiagnosed for years. Currently, inherited platelet diseases involve multiple rare monogenic disorders from mutations in more than 60 specific diagnostic-grade genes.
Analysis of platelet production and size plays a crucial role in guiding the differential diagnosis.
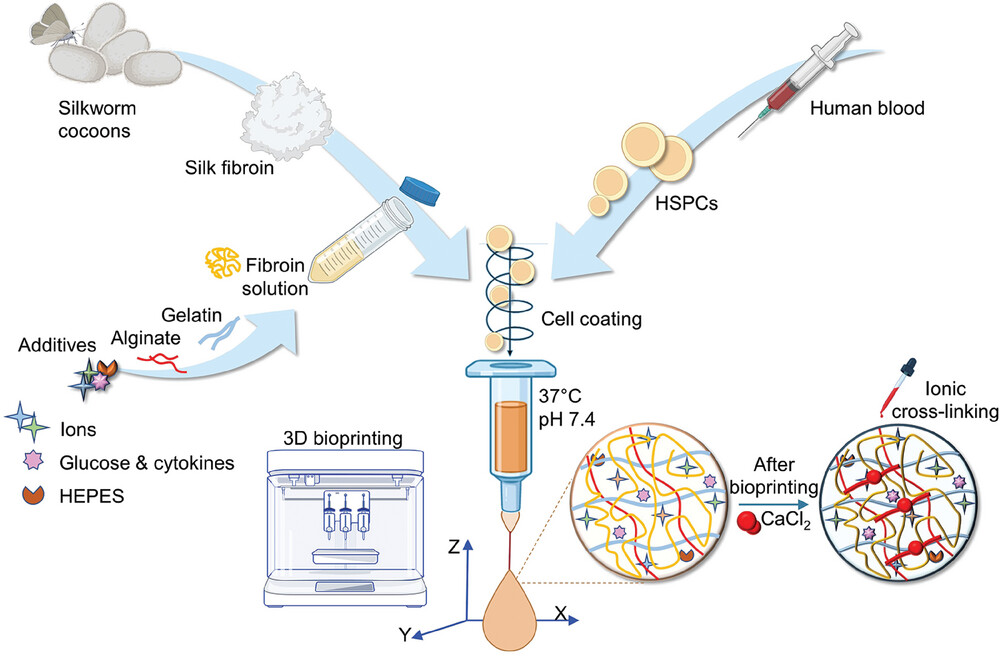
HSPCs (human pluripotent stem cells) are fragile and require a defined chemical-physical and mechanical environment to survive and differentiate outside the human body. A tailor-made bioink was designed to support the bioprinting of blood progenitor cells under physiologically relevant conditions (37 °C, pH 7.4) and provide a stable biomimetic 3D microenvironment that supports their survival, differentiation and maturation. Specifically, a solution of regenerated silk fibroin, sourced from natural Bombyx mori silkworm cocoons, was mixed with gelatin and sodium alginate and, to provide fuel for cellular metabolism, the system was fed glucose.
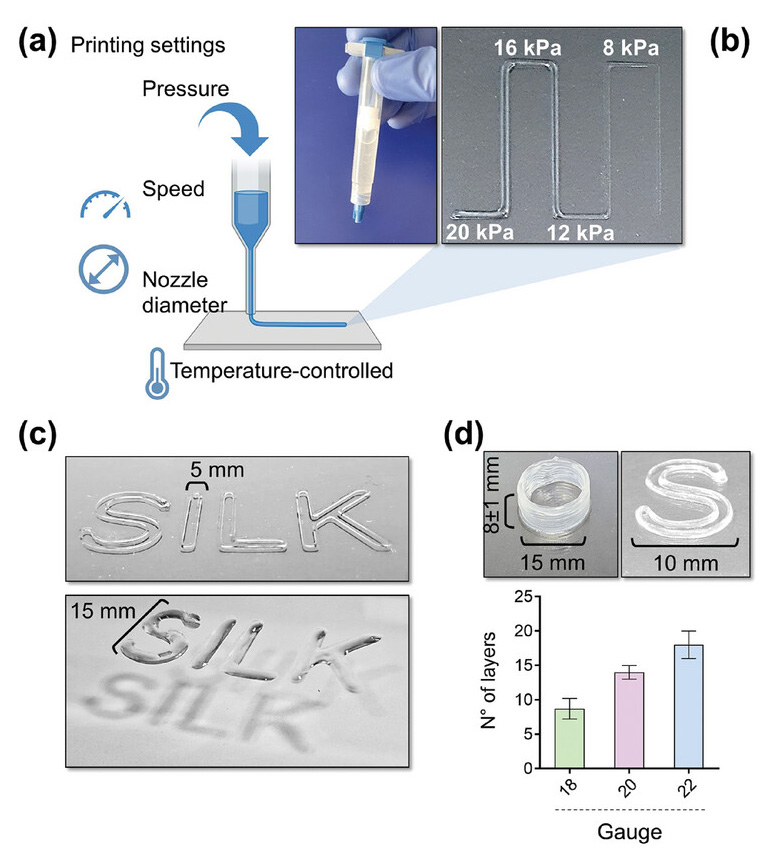
The ink was extruded through a head heated to 37 °C and placed on a cold print bed (<25 °C) to initiate thermosetting, at a speed of 8-10 mm/s, pneumatic pressure of 12-16 kPa and through a nozzle of 20G. The gelled 3D constructs were immediately immersed in a saline buffer solution containing CaCl2 to cross-link the alginate. The gelled 3D constructs were soon after immersed in a saline buffer solution containing CaCl2 to cross-link alginate.
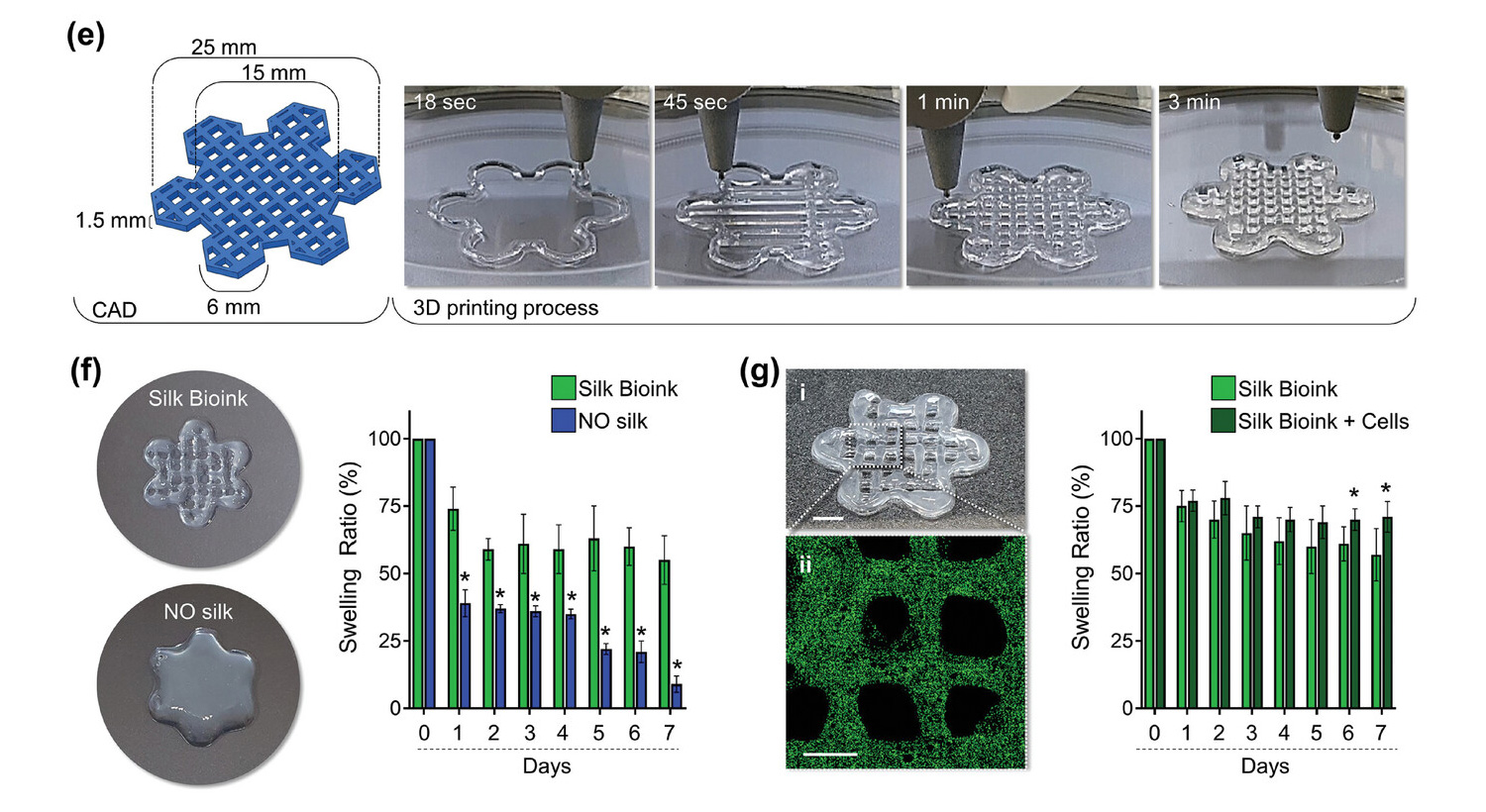
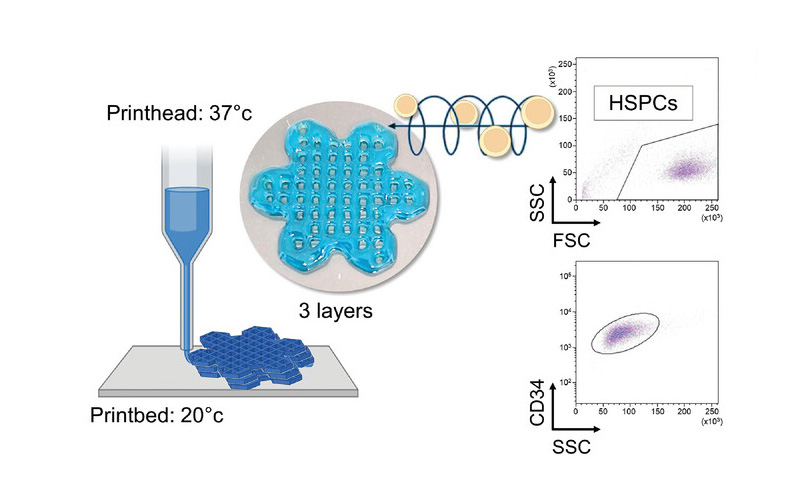
The 3D printed model represents a layered hexagonal grid that intersects smaller hexagons at its edges to simulate a flower-like structure that mimics the microvascular network to facilitate the exchange of nutrients, oxygen and waste products within the printed tissue, enhancing thus cell survival and function.
The shape of the printed constructs was found to be highly consistent and matched to the input values with excellent extrusion consistency and structural stability throughout the printing process, a result in stark contrast to the silk-free bioink, which fused rapidly after printing. deposition, resulting in fusion of the layers to form structures of poorly defined characteristics.
Confocal microscopy reconstruction showed that the cells were distributed homogeneously in the 3D structure and the integrity of the silk bioink was maintained during culture, without major changes in the swelling ratio.
Live/dead staining, performed immediately after bioprinting, revealed a highly viable population. Furthermore, a custom-made buffer was developed to dissolve the 3D construct. When HSPCs were recovered from the dissolved construct, cell viability was well preserved, with no significant differences over time (day 2 (93 ± 3%) and day 7 (93 ± 4%)).
3D imaging demonstrated a living cellular microenvironment in which cells, when cultured in the presence of thrombopoietin, transformed from diploid progenitors to polyploid megakaryocytes, phenotypically analogous to those observed in native bone marrow tissue. An in-depth flow cytometry analysis of the cells, recovered from the silk bioink, showed the expression of the entire spectrum of megakaryocytic surface markers: more than 85% of the cells express CD29, CD41, CD42a, CD42b, CD49b , CD49d and CD61. The transcript of genes for extracellular matrix components (e.g., fibronectin, collagen type III, and collagen type IV) and matrix remodeling enzymes (e.g., MMP2 and MM9) is increased over the course of maturation, demonstrating the physiological adaptation of cells that faithfully replicated the differentiation process of megakaryocytes as occurs within the human body.
High-resolution imaging at the single-cell level demonstrated that, during maturation, megakaryocytes undergo a substantial shape change, from a regular round morphology to irregular shapes that invade the 3D microenvironment. Volumetric analysis of 3D culture established that low-volume progenitors, showing a high mean sphericity index, transform into large megakaryocytes, which significantly increase their volume by losing sphericity in favor of highly branched margins, reminiscent of the formation of pro-platelets.
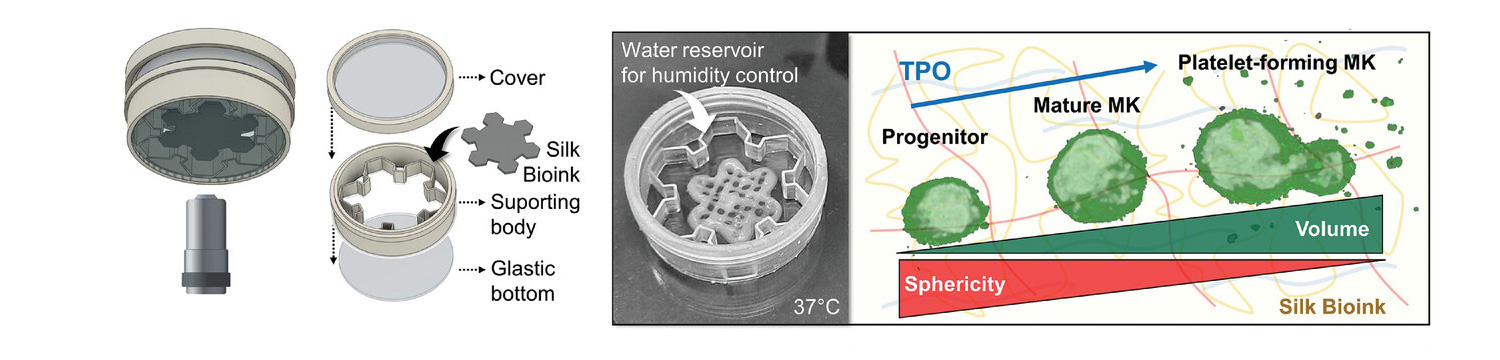
Bone marrow megakaryocytes are known to synthesize lipids and cholesterol that provide fluid membranes to elongate pro-platelets. 3D reconstructions of cells showed that pro-platelets, enriched in cholesterol along their length, thin across 3D space and actively release platelet-sized particles with CD41a+ and CD42a+ markers.
This bioprinting approach was applied to megakaryocytes derived from patients who had previously been diagnosed with MYH9-related disease (RD) and ANKRD26-related thrombocytopenia (RT), two forms of hereditary thrombocytopenia characterized by moderate to severe low platelet counts.
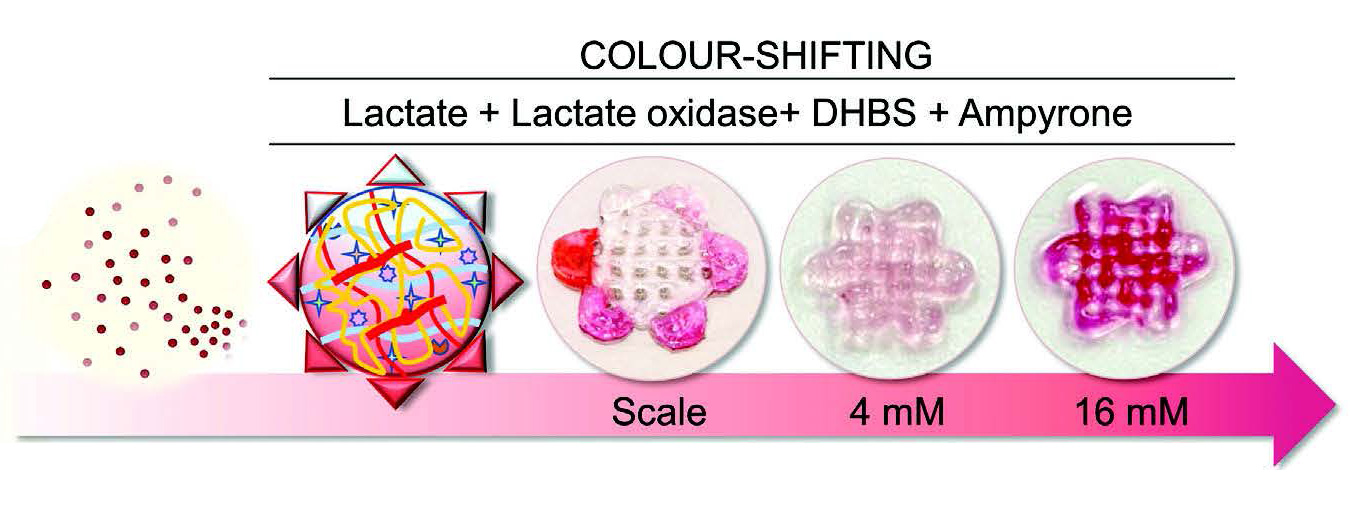
Furthermore, enzymatic reactions in the silk bioink were incorporated directly into the formulation to track cellular metabolism via colorimetric quantifications that can provide a standardized, rapid and non-destructive measurement of cellular function and platelet production in the 3D construct. Lactate level was chosen as the test indicator because of the intimate connection between megakaryocyte glucose metabolism and platelet generation. The glycolytic pathway and the Krebs cycle play a crucial role in supporting lineage specification, nuclear multiplication, and membrane accumulation necessary for the successful transformation of megakaryocytes into functional platelets by facilitating the synthesis of glycoproteins, nucleotides, and lipids. HRP was incorporated into the silk bioink formulation to evaluate the stability of the enzyme in its 3D printed format. Constructs were stored at 4, 37, or 70 °C for 15 days, and comparable chemiluminescence emission demonstrated the ability of the silk-based bioink to preserve overall enzymatic activity.
In conclusion, a comprehensive approach for 3D bioprinting of human blood progenitor cells in a standardized and automated manner has been developed. This methodology facilitated the creation of 3D models characterized by highly reproducible mechanical properties, structural integrity, and uniform cell distribution. These advances have improved megakaryocyte function, leading to improved platelet production.
This knowledge can contribute to a deeper understanding of underlying disease mechanisms, guide the prioritization of drug development strategies, and pave the way for high-throughput screening of new therapeutic options directly on patients' blood progenitors.
Source: PubMed Central