Non-invasive technique allows screening of thousands of tumor organoids at the level of individual cell populations, including those from which drug resistance arises.
To test in vitro cancer drugs, researchers are starting to use three-dimensional avatars of tumors that grow inside the body. But these "tumoroids" do not exactly reproduce the diversity between the cell populations that make up the tumor, often at the origin of drug resistance phenomena or the appearance of metastases. Thanks to a non-invasive technique based on interferometry, a group from the UCLA Jonsson Comprehensive Cancer Center (California, United States) was able to analyze the response to drugs of various cell sub-populations simultaneously in thousands of 3D printed tumoroids without destroying them, with a series of measurements repeated over time. The results were published on Nature Communications.
A tumoroid derives from tumor tissue samples taken from cancer patients, which researchers are able to grow in the laboratory in conditions similar to physiological ones. Like organoids, miniature replicas of organs made in the laboratory, so too tumoroids recapitulate the three-dimensional spatial organization of tumors growing in the human body. These models constitute a valuable investigative tool for studying tumors in vitro, without the limitations of classic two-dimensional cell cultures or animal models which, despite being three-dimensional, have different from the human being and a higher cost.
Tumoroids allow testing in vitro the effect of drugs on an "avatar" of the tumor that grows inside the body. This possibility opens up new scenarios in the panorama of precision medicine, because it allows you to know in advance whether or not the patient's tumor will respond to treatment and to establish a personalized treatment plan. But many of the potential of this technology have not yet been exploited. A big problem to solve is the ability of these models to reproduce intra-tumor heterogeneity, i.e. the diversity between the cells of a single tumor. It would be a mistake to consider the tumor as a single, homogeneous mass: much more often, however, it contains different cell populations, some more sensitive to therapies and others more resistant, thanks to which many tumors resist treatment or form aggressive metastases.
In in vitro models, the challenge is not only to reproduce the diversity of cellular populations, but also to monitor the response to drugs over time, which can produce changes in the number, shape, or organization of these cells. In other words, researchers should be able to capture images of the interior of the tumoroid, without altering or destroying its three-dimensional structure. But most of the methods of analysis are of the destructive type, i.e. they do not keep the structure intact for subsequent measurements. Furthermore, the resulting information is only an "average" of the characteristics of the cell populations that make up the organoid: it represents the majority, but does not capture the small differences between one cell and another.
Researchers at the UCLA Jonsson Comprehensive Cancer Center have therefore studied a new imaging system based on high-speed cell interferometry (HSLCI), a very high resolution non-destructive approach, which allows also characterize individual drug-resistant populations. Interferometry uses light as a sensitive measuring instrument: a laser is aimed at the target and an optical sensor measures the displacement of the light transmitted through the sample with respect to the original. This technique makes it possible to calculate the mass density of the tissue in real time, which correlates directly with the biosynthetic and degradative processes that take place inside the cells.
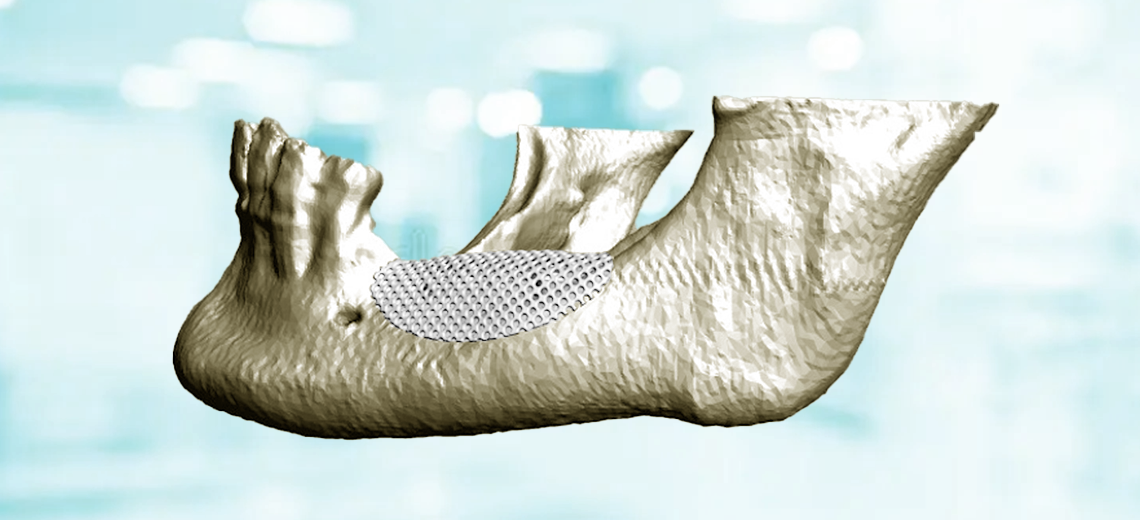
Researchers have used the 3D bioprinting technique to produce tumor organoids that are as similar as possible to real tumors. A thin layer of cells was "printed" on a base of extracellular proteins, reproducing the histological characteristics of the original tissue without altering gene expression. Thanks to the HSLCI technique the researchers were able to simultaneously measure the masses of thousands of 3D printed tumoroids. These measurements do not damage or destroy the organoids, thus allowing a non-invasive analysis of the growth of individual cell populations over time, before and after treatment with different drugs. Even within very homogeneous samples, the team identified small groups of cells that, unlike the majority, do not respond to therapies: these resistant nuclei are often the ones that give rise to recurrences or metastases, because they are not eliminated by first-line treatments .
This technology may be important for studying drug resistance mechanisms and speed up screening activities. Within hours, researchers were able to identify which organoids are sensitive or resistant to certain therapies: the hope for the future is to use these models as patient avatars, to select the best treatment option for each.
source: Osservatorio Terapie Avanzate