The term brachytherapy denotes a special radiotherapy technique (from the Greek “brachy”, “short”) in which radioactive sources are placed in or near the tumor tissue, which is why it is also called internal or direct contact radiotherapy.
In brachytherapy, radiation doses are delivered over small distances with high dose gradients to specifically target the tumor tissue while minimizing damage to surrounding healthy tissue.
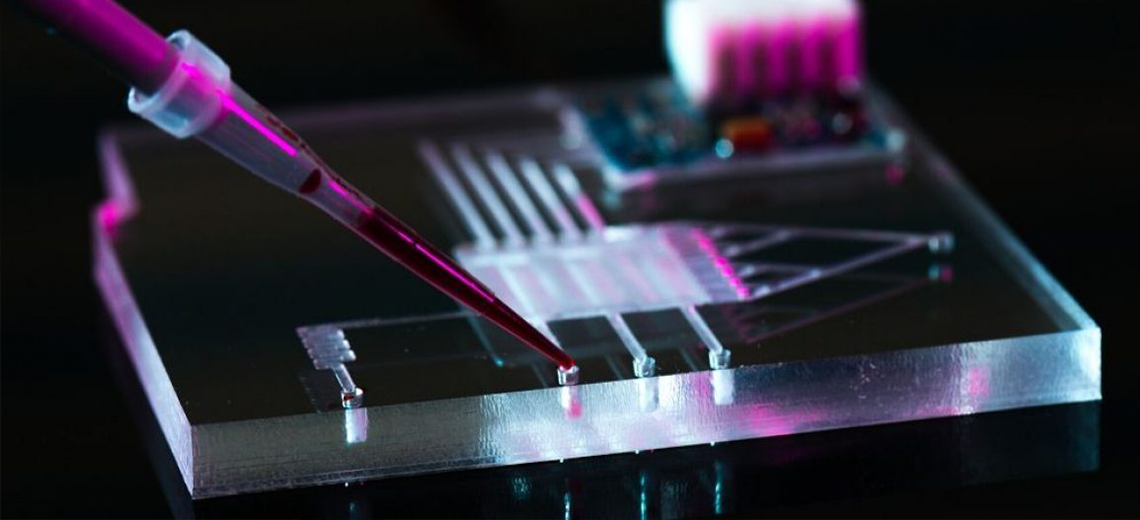
Brachytherapy treatment involves placing very small radiation sources typically consisting of the radioisotope Iridium 192 (Ir192) very close to the cancerous area (Fig.1). When the radioisotope used releases high dose rates as in the case of Ir192, brachytherapy is referred to as HDR (High Dose Rate).

Figure 1: source of Ir192
Each brachytherapy technique, depending on the anatomical structure to be irradiated, involves the use of devices, called applicators, whose purpose is to convey and house the radioactive source at the treatment site and to maintain its correct position throughout the duration of therapy.
When brachytherapy is used for the treatment of extensive skin lesions, the standard applicator used today is the Freiburg Flap: a flexible applicator that consists of a mat composed of small spheres (5 mm radius) made of silicone characterized by a medial channel for the passage of the radioisotope inside it through specific catheters (Fig.2).
For each patient, this mat is shaped ad-hoc according to the geometry of the anatomical site to be treated; however, due to the size of the spheres it has poor adherence to the skin in the case of anatomical structures with complex curvatures (nose,lip,ear) and provides a fixed (5mm) and non-adjustable distance between the radioactive source and the patient's skin. The preparation of the mat for each specific skin treatment also requires management by a properly trained experienced staff.
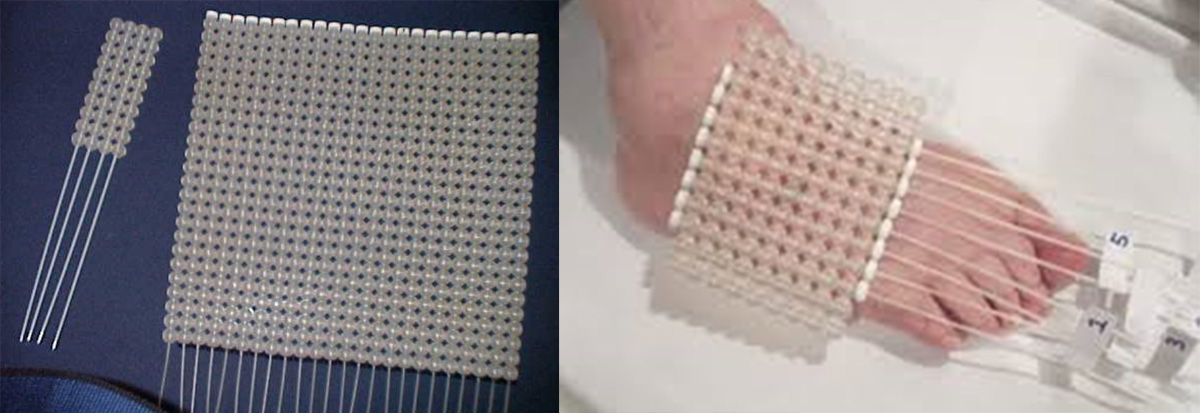
Figure 2: (left) Freiburg flap mat equipped with catheters; (right) example of preparing the Freiburg mat for skin treatment of a limb
Poor adherence of the mat to the patient's anatomical surface can lead to the presence of air voids, which can compromise the reproducibility of the treatment across sessions and inadequate delivery of the designated dose to the target.
All this has highlighted the need for fully customized HDR skin brachytherapy applicators for each patient, which allow high adherence to the surface to be treated even on anatomies with complex curvatures, high reproducibility in positioning, a customized definition of catheter paths in terms of spacing and distance from the skin for optimal treatment success.
3D CAD design and additive manufacturing (AM) technologies present themselves as a valuable tool for modeling and creating custom applicators.
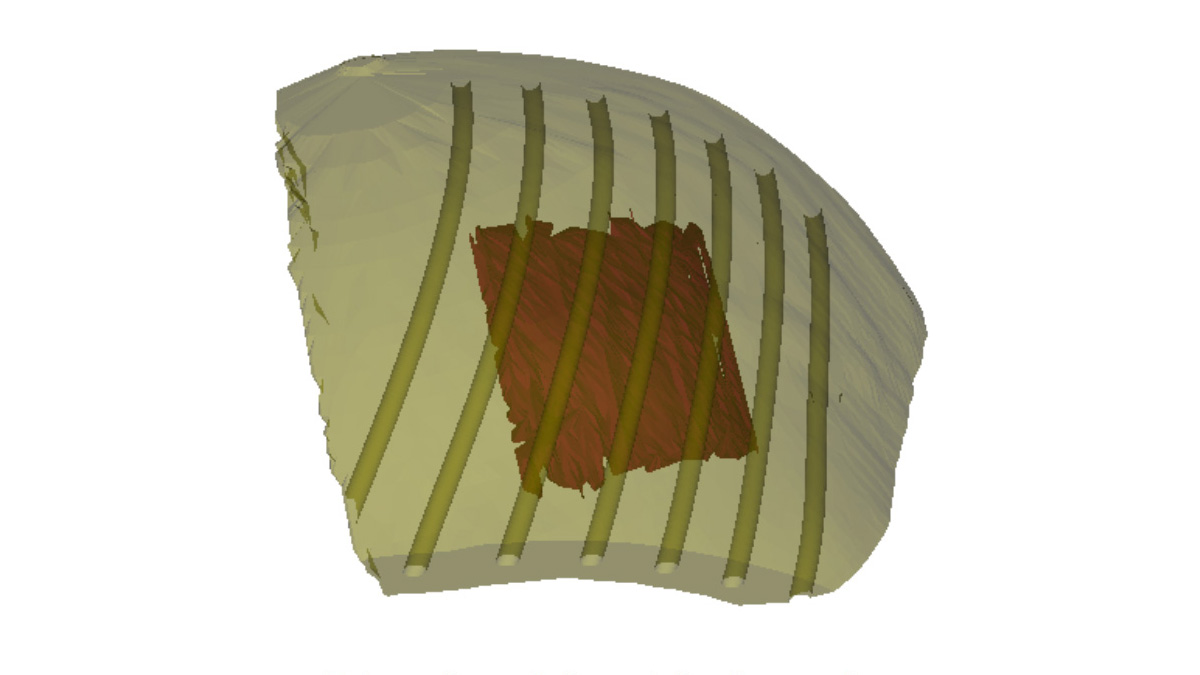
Figura 3: esempio di applicatore per brachiterapia cutanea HDR modellizzato tramite software CAD con appositi percorsi per cateteri ottimizzato per irradiare il target evidenziato in rosso
The Department of Medical Physics and Radiotherapy at the “S.G. Moscati” Hospital in Taranto in collaboration with the CreaMED team has chosen to use a fully digital workflow for the design (Fig.3) and fabrication of HDR skin brachytherapy applicators, using specific CAD segmentation and design software and FFF printing technology with the UltiMaker S5 3D printer with TPU 95A flexible filament (Fig.4).

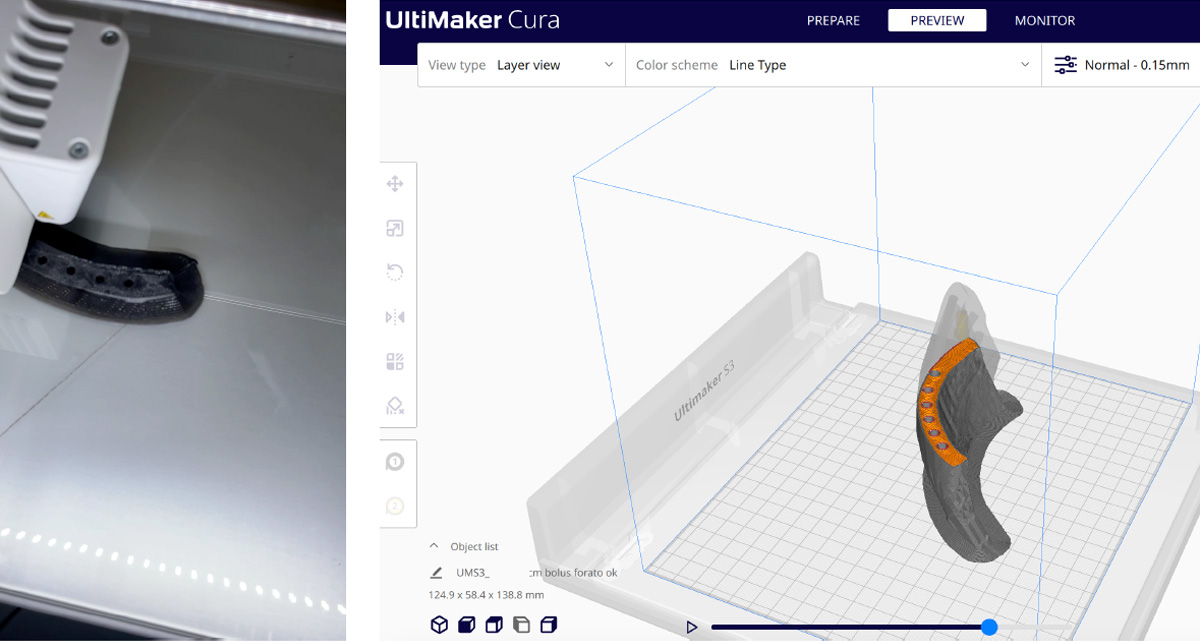
Figure 4: Ultamaker S5 3D printer and 95A TPU filament used for printing the custom HDR brachytherapy applicator suitably molded for optimal treatment.
The results of this project were presented at the 12th AIFM (Italian Association of Medical and Health Physics) National Congress 2023 highlighting all the advantages found over a conventional Freiburg Flap, the limitations of the technology, and future goals aimed at improving the workflow.
The applicator was studied from a geometric and dosimetric point of view.
The geometric characterization of the applicator consists primarily of a visual assessment of whether the part is printed correctly if there are errors in layer deposition or air voids. To remedy this, a 100% density infill with concentric geometry was set in the slicing software. Next, the adhesion of the printed part to the surface of the patient/puppet was evaluated.
Dosimetric characterization, on the other hand, involves comparison of the planned dose with the delivered dose, performed by analysis of radiochromic films in a simplified irradiation setup.
Comparing the classic Freiburg Flap mat with a specific 3D-printed applicator, geometrically, it is observed that with the 3D-printed applicator there is an important reduction in air volumes between the patient surface and the applicator, as can be seen from the analysis of the tomographic images (CT images) acquired for comparison (Fig.5)
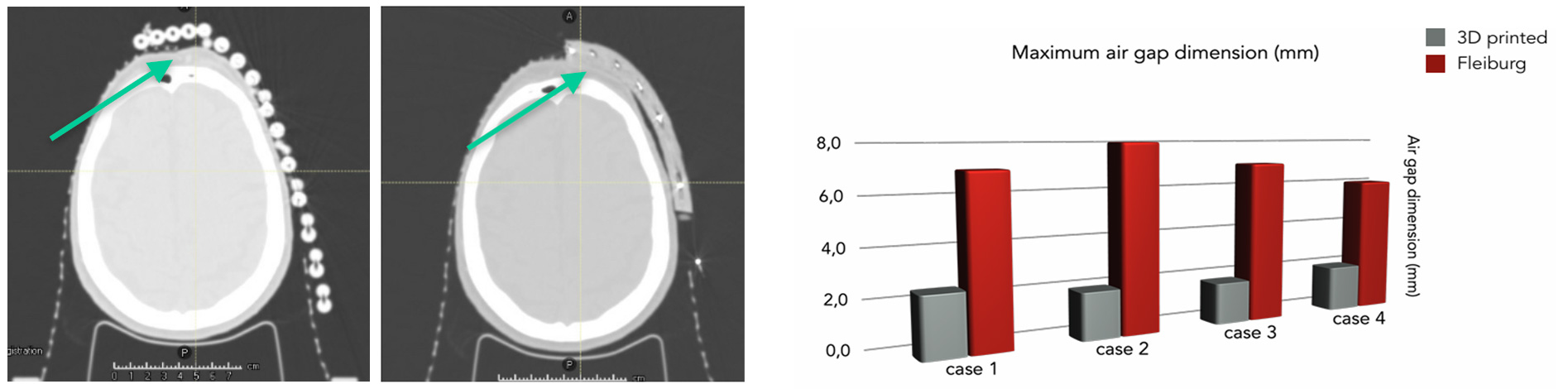
Figure 5: Geometric characterization of 3D printed HDR applicator for skin lesion treatments compared to Freiburg standard
From a dosimetric point of view, the 3D applicator also shows advantages in terms of covering the skin lesion and sparing critical organs located nearby when compared with a standard Freiburg applicator (Figure 6).
The TPU used to make the applicator also has a density close to that of water: this is a fundamental requirement to ensure the correct application of the TG 43 formalism on which the calculations for the realization of the brachytherapy treatment plan are based.

Figure 6: Treatment planning for covering the same lesion using a 3D printed applicator (left) and a standard Freiburg mat (right) in comparison
This study highlighted that the 3D printed applicator is geometrically accurate, provides maximum customization of therapy, moderate fabrication time with print times between 2 and 11 hours depending on the size of the region to be treated, high reproducibility of the setup, and improved ergonomics for the patient.
However, some limitations also emerged from this study that lay the groundwork for future challenges in anticipation of improving clinical implementation for complex cases, such as identifying new materials that are more flexible for better adherence even in the case of anatomical sites with complex curvatures and the possibility of combining an in vivo dosimetry system into the applicator.
Source: Congresso AIFM 2023