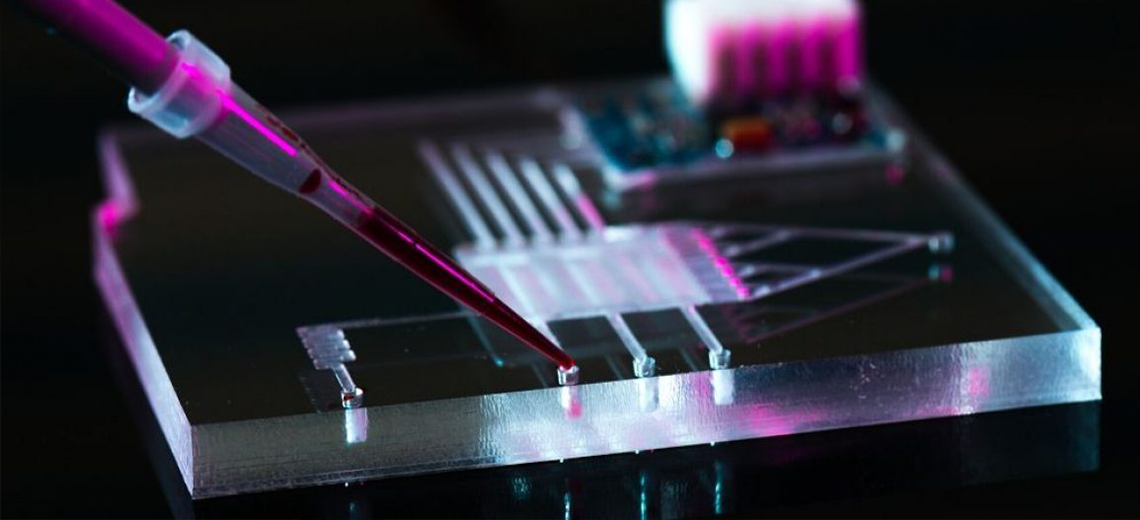
Microfluidics is an interdisciplinary field of science and technology that deals with the highly precise control and manipulation of fluids by geometrically constraining them in networks of small channels, typically less than 100 µm in diameter.
Compared to traditional testing methods using Petri dishes and droppers, microfluidics uses considerably smaller sample sizes, requiring much less expensive chemicals and reagents. Several interesting properties emerge when dealing with such small amounts of fluid. Properties such as surface tension, which tend not to matter when dealing with the volumes of water we are used to, start to dominate the dynamics at these scales. The Reynolds number, which determines the turbulence of the flow, is extremely low at small scales, meaning that the fluid flow remains virtually laminar. All of this makes microfluidic-based testing safer, because it allows toxicants to be controlled and contained more effectively.
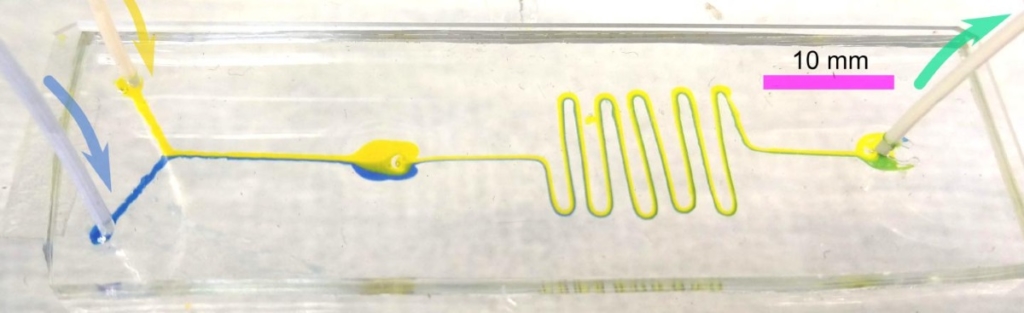
Applications
Microfluidics has become increasingly important in the development of new technologies in medicine, biology, physics, chemistry, and engineering.
In biotechnology, microfluidics is used for sample preparation, cell culture and analysis, and drug delivery. In medical diagnostics, it is used for point-of-care and lab-on-a-chip systems. In chemical and materials sciences, microfluidics can be used to synthesize and study nanomaterials. In engineering, it is used for process optimization and control. All fluids must be relatively pure and free of particles that clog these delicate channels. DNA microarrays, which allow biologists to simultaneously run millions of tests on a particular protein or gene sequence, use microfluidics. Chemical separation machines can use combinations of centrifuges and microfluidic chips to analyze the chemical composition of a particular substance. They can be used to prepare biological samples for testing. Since most microfluidic chips have designs that cannot be reconfigured, this limits more ambitious applications, but research is ongoing to circumvent this problem.
Microfluidic Systems
Microfluidic systems are typically composed of several components, including fluidic channels, pumps, valves, sensors, and actuators. Microfluidic system components are typically fabricated using soft lithography or 3D printing techniques.
There are three main types of microfluidic systems: lab-on-a-chip systems, point-of-care systems, and microfluidic reactors. Lab-on-a-chip systems are used for laboratory-scale experiments, while point-of-care systems are used for patient diagnosis and monitoring. Microfluidic reactors are used for chemical and biochemical processes.
Fabrication of microfluidic chips
Systems using microfluidics must be fabricated very precisely. Glass is a common material, but plastic and silicon are also popular mediums. Traditional approaches to fabricating microfluidic devices tend to be complex and time-consuming, typically involving cleanroom microfabrication of a master from a 2D photomask followed by soft lithography and bonding. This ubiquitous protocol, however, introduces significant costs associated with institutional infrastructure, equipment setup, maintenance, and the physical space required for cleanroom facilities. This limits the accessibility of microfluidic technologies for many research laboratories. Additionally, each design iteration requires printing a new photomask and UV lithography to produce a new master. This multi-step, labor-intensive process currently hinders rapid, widespread innovation and the development of new applications.
Among the manufacturing techniques used in particular are:
- Photolithography
- Micro thermoforming
- Micro injection molding
- CNC machining
- 3D printing
3D printing unlike other manufacturing techniques that operate by subtraction and etching, works by addition of material. In the last decade, a wave of technological advances in high-resolution 3D printing has further improved our ability to fabricate microscale structures and microfluidic devices. Using this technology, microfluidic devices can be printed in different ways, as explained below.
PDMS-bsed microfluidic mold fabrication
PDMS-based microfluidic fabrication using 3D printed master molds offers many intuitive advantages of 3D printing fabrication and maintains the desirable properties of PDMS material, such as biocompatibility and oxygen permeability. This approach also overcomes the disadvantage of unknown surface properties of commercial resins. The master can be produced via rapid prototyping techniques, reducing the overall cost and time required for fabrication, followed by traditional PDMS molding.
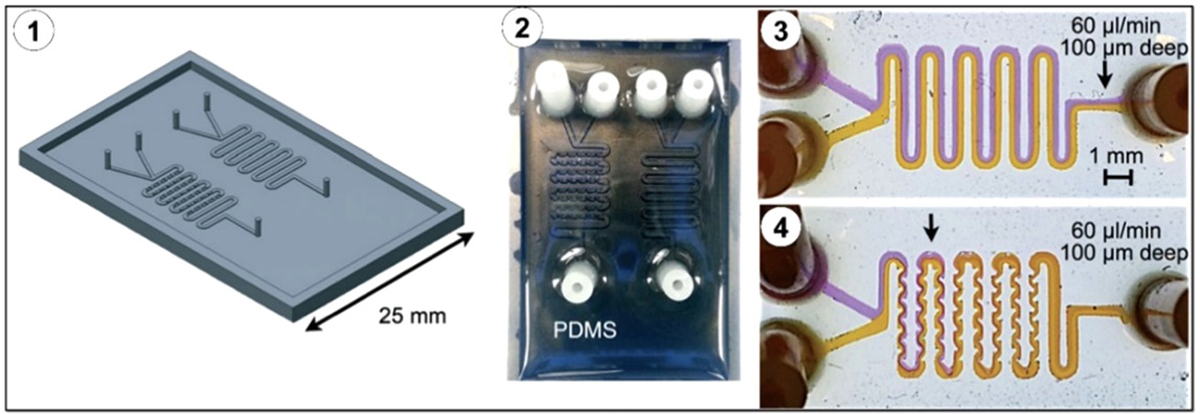
Fabrication of microfluidic components
Fabricating microfluidic devices with precise, microscale channel geometries typically requires a significant amount of work in terms of design, fabrication, and integration of microfluidic components, including active and passive components. Active components manipulate fluid flow by applying energy, while passive components rely on external actuation forces or capillaries to drive fluid flow.
In addition, microfluidic modules can be fabricated separately and then combined to achieve an interchangeable design that can be easily adapted to perform the desired function.
In both approaches, the use of 3D printing technology replaces cleanroom microfabrication steps, simplifying the fabrication of complex devices and reducing initial investment and operating costs compared to traditional fabrication.
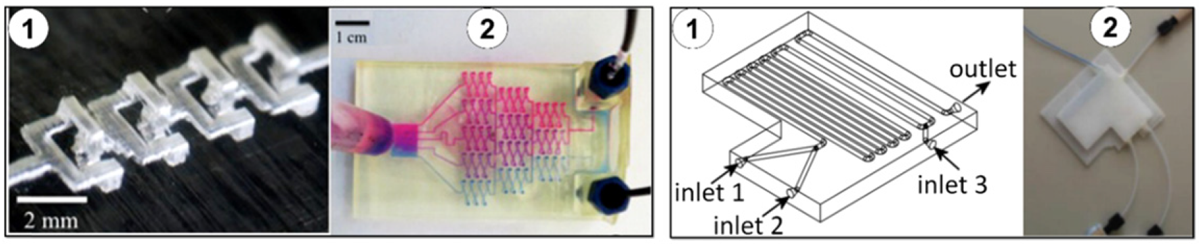
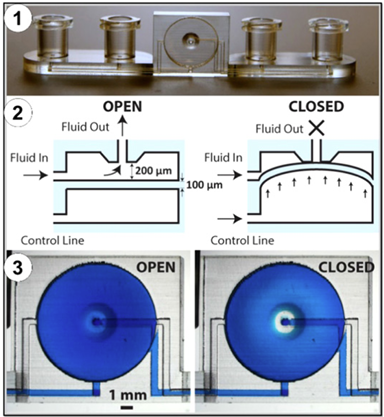
Properties of 3D printed materials
It is therefore essential to understand what characteristics the microfluidic device must satisfy to identify the most suitable technology and material. The elastic modulus is the mechanical relationship between stress and strain, which is of utmost importance in the design of parts such as channels and valves that must withstand high fluid pressure. Although microfluidics handles such small amounts of fluid, the pressure generated by pumping through these devices can be sufficient to cause elastic deformation in the channel or valve that is integral to regulating the microfluidic process. In microfluidic applications, the transparency of the materials is also a useful property to consider. In microfluidics, visual observations are necessary to ensure that the reagents are interacting correctly and flowing appropriately. Additionally, in biological applications of microfluidics, cells can be visualized using transmitted light or epifluorescence.
However, it should be noted that due to the light curing method involved in SLA 3D printing, especially in the case of transparent resins, there may be an issue with unintended curing layers above the layer being printed.
Additionally, resin viscosity is an important parameter in choosing an appropriate method for removing uncured resin from 3D printed channels.
Microfluidic applications often involve biomaterials or chemical reagents. The chemical composition of the resin is critical to making 3D printed devices a viable option. Devices must be made of resins that do not react with or absorb protein and nucleic acid reagents to be useful in biomedical applications. Likewise, resins must be biocompatible, as living cells may meet or be cultured near the resin. The biocompatibility and transparency of SLA and multi-jet resins have been shown to be sufficient for biological experiments. Given the highly favorable properties of existing resins and the active innovation in this field, the use of 3D printing materials to create microfluidic devices has gained popularity.
3D printing of microfluidic devices for use in various research settings has several advantages over existing microfabrication tools, including accessibility, low cost, efficiency, versatility, and rapid prototyping. Several challenges remain, including the choice of materials and limitations on the smallest channel sizes that can be fabricated. High resolution and precision in microfluidic devices have been achieved with photopolymer jetting 3D printers. However, other techniques such as binder jetting and laser sintering offer the advantage of not requiring fixed support structures, which can be difficult to remove after the photopolymer jetting process.
Source: PubMed